In the same section
-
Share this page
Protein Tyrosine Phosphatases-mediated prognosis of obesity-induced hepatocellular carcinoma [Technology Offer]
The technology in a nutshell
Novel diagnostic and prognostic assay relying on the expression and oxidation levels of Protein Tyrosine Phosphatases (PTPs) of Hepatocellular carcinoma (HCC).
State of the art
Liver cancer is the fourth most common cause of cancer death worldwide with HCC, which is refractory to nearly all currently available anti-cancer therapies, accounting for 90% of such primary cancers. The incidence of HCC is rapidly increasing in economically developed countries and is mostly attributable to alcoholic, and non-alcoholic fatty liver disease (NAFLD). Obesity, which affects more than 650 million individuals worldwide, is a well-established risk factor for NAFLD which can in turn progress to fibrosis, cirrhosis, non-alcoholic steatohepatitis (NASH) and HCC.
With an increasing number of patients developing NASH-related liver disease and pharmacological treatments on the horizon, there is a pressing need to develop HCC biomarkers for prognosis and selection of patients for treatment and monitoring.
The invention
Protein Tyrosine Phosphatases (PTPs) are responsible for the modulation of protein tyrosine kinase activity through dephosphorylation of tyrosine residues and are thereby key regulators of ligand-mediated receptor signaling and cell cycle events. Loss of the tight regulation in protein phosphorylation results in over- or under-activation of key signaling pathways implicated in the pathogenesis of various diseases, including obesity, steatosis and HCC.
The inventors have developed a novel diagnosis and prognosis proteomic assay based on PTPs activity. The measurement of aggregate expression and oxidation levels of PTPs in liver biopsies indeed enables the diagnosis, prognosis and monitoring of disease progression from steatosis -> NAFLD -> NASH to HCC.
The assay also provides means for selecting the most appropriate therapeutic treatment options according to the disease progression and monitoring the response to the corresponding therapy, enabling the development of personalized therapeutic strategies to improve survival. The combined measurement of expression levels of specific individual PTPs and oxidized PTPs provides additional means to fine-tune patient stratification.
Key advantages of the technology
- Proteomic assay enabling diagnosis and patient stratification of according to stage of transition from steatosis/NAFLD/NASH to HCC from human biopsies.
- Robust Mass-Spectrometry method enabling direct analysis of biopsy samples also conducive to the development of blood-based biomarker assays, including ELISA.
Potential applications
- Proteomic assay enabling NAFLD/NASH/HCC diagnosis, prognosis and patient stratification for therapeutic treatment decision-making
- Companion diagnostic for assessing treatment efficacy including innovative approaches under clinical development
- Possible implementation as blood-based assay suitable for clinical settings
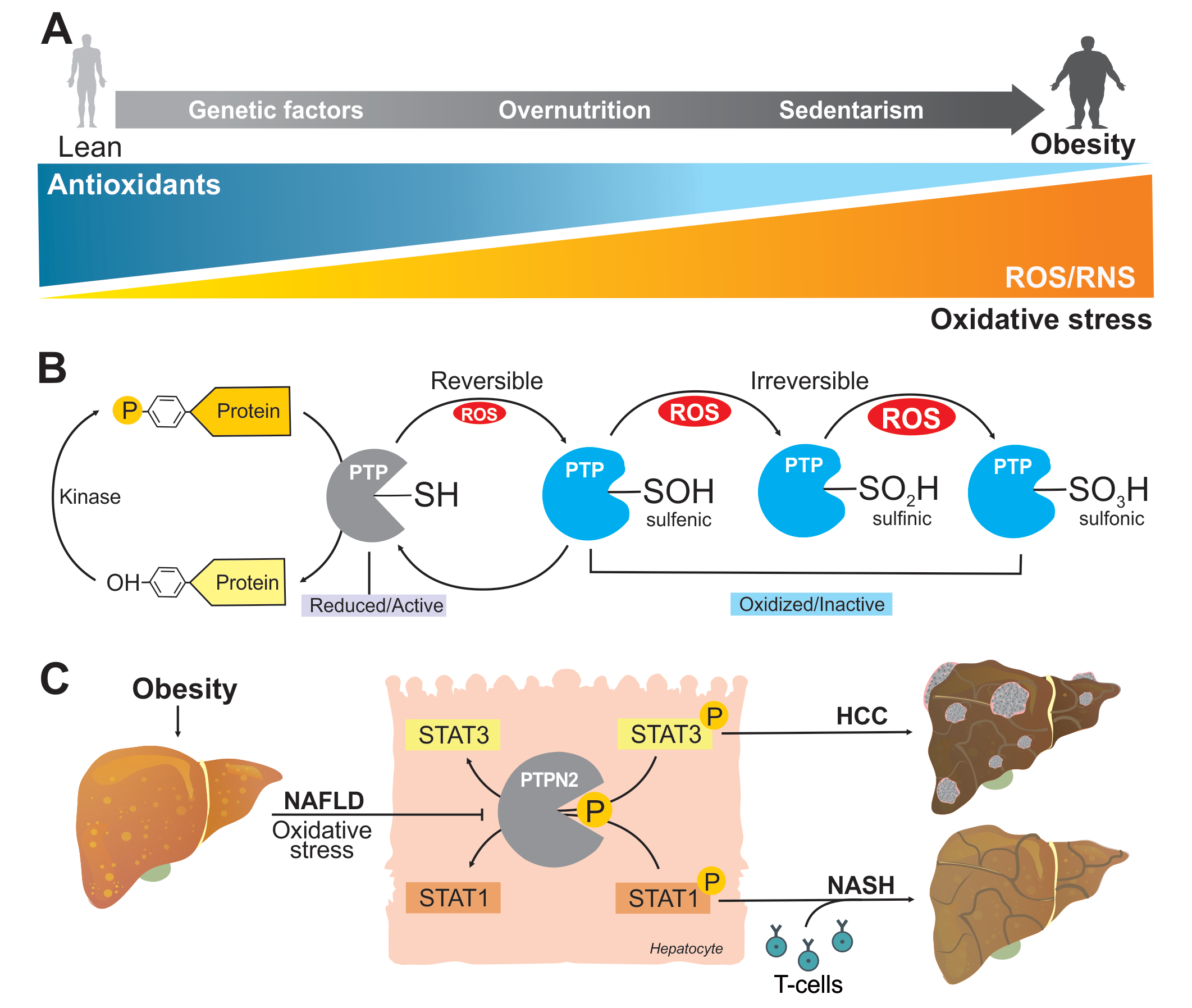
Obesity contributes to the development of NASH and HCC.
A. One of the consequences of obesity is an unbalance between the production of oxidant species and antioxidant capacity leading to oxidative stress generated by reactive oxygen and nitrogen species (ROS and RNS).
B. PTPs regulate tyrosine phosphorylation-dependent signal transduction through tyrosine dephosphorylation of protein substrates. The protein structure and presence of cysteine residue in the active site of PTPs render them highly susceptible to oxidation by ROS to the reversible form of sulfenic acid or irreversible forms of sulfinic and sulfonic acid accompanied by conformational changes that inhibit PTP activity and prevent substrate binding.
C. Obesity is frequently associated with NAFLD, a condition that favors liver oxidative stress. Under this condition, STAT1 and STAT3 phosphatase protein tyrosine phosphatase non-receptor type 2 (PTPN2) is inactivated, thereby increasing STAT1 and STAT3 signaling. While heightened STAT1 signaling is responsible for the recruitment of activated cytotoxic T cells and ensuing NASH and fibrosis, this is not essential for HCC. Rather, STAT3 signaling promotes HCC without NASH and fibrosis.
Technology Readiness Level

TRL-3 Proof of concept assay established for the Mass-Spectrometry method – Clinical validation ongoing.
The team
Prof Esteban Gurzov has dedicated his scientific career to investigate the pathogenic mechanisms of obesity and diabetes and the discovery of novel therapeutics. In 2017, he received a tenured Associate Researcher/Chercheur Qualifié position from the FRS-FNRS to direct the Signal Transduction & Metabolism Laboratory. In 2019, he was awarded with a European Research Council (ERC) Consolidator Grant. He is also a WELBIO Investigator since 2019 and was recipient of one of the 2021 AstraZeneca Foundation Award.
The Signal Transduction & Metabolism Laboratory, located on the academic hospital campus aims at translating research discoveries to the clinic through a combined use of molecular biology, stem cells research, animal models of metabolic disorders and human samples.
Relevant publication
- Oxidative stress in obesity-associated hepatocellular carcinoma: sources, signaling and therapeutic challenges, Brahma M.K., Gilglioni E. H., Zhou L., Trépo E., Chen P., Gurzov E. N., Oncogene, 40: 5155-5167, July 2021 - DOI: 10.1016/j.cmet.2014.05.011
- Hepatic Oxidative Stress Promotes Insulin-STAT-5 Signaling and Obesity by Inactivating Protein Tyrosine Phosphatase N2, Gurzov E. N.,Tran M., Fernandez-Rojo M. A.,Merry T. L., Zhang X., Xu Y., Fukushima A, Waters M. J., Watt M. J., Andrikopoulos S., Neel B. G., Tiganis T., Cell Metabolism, 20: 85-102, July 2014 - DOI: 10.1038/s41388-021-01950-y
Keywords
- Oncology
- In Vitro proteomic Diagnosis/Prognosis
- Hepatocellular carcinoma (HCC)
- Obesity
Collaboration type
- Licence agreement
- R&D collaboration
Inventors
Esteban Gurzov
Wu Wei
Contact
ULB Research Department
Fred Pierard
IP Manager
+32 (0)2 650 32 26
frederic.pierard@ulb.be