Dans la même rubrique
-
Partager cette page
Modified-release 3D printed capsules [Offre de technologie]
The technology in a nutshell
New method for the production of functionalized capsules by 3D printing used to modify the release of drugs.
State of the art
In pharmaceutical companies, coatings are currently performed on both tablets and capsules using fluid bed coaters or perforated pan systems. In contrast, in compounding pharmacies, hospitals, or when small batches are required to carry out pre-clinical studies, coatings are often achieved by dipping. The efficacy of this coating method is considered to be erratic and may lead to potential therapeutic failure.
It has long been known that the main zone of weakness of capsules, which can create a non-sealing system, is the point of contact between the body and the head, regardless of the presence or absence of a coating. Nevertheless, it was also demonstrated that the domes at both extremities of the capsule as well as the permeability of the wall itself, may be considered as a secondary potential cause of water diffusion prior the dissolution of the capsule shelf.
The invention
The invention describes the production of functionalized capsules by 3D printing to modify the release of drugs. It proposes to substitute the commonly used dipping method of coating in compounding pharmacies, hospital and preclinical studies by a 3D printing method of production as the efficacy of such coating method is known to be erratic and may lead to potential therapeutic failure.
The invention relates to 3D printing techniques which represents an elegant tool for designing simple, accurate, cheap, structured and tailored drug delivery systems in pharmaceutics. Here, 3D printing is used to produce unique modified-release (e.g. enteric, colonic, sustained release) capsules ready-to-use.
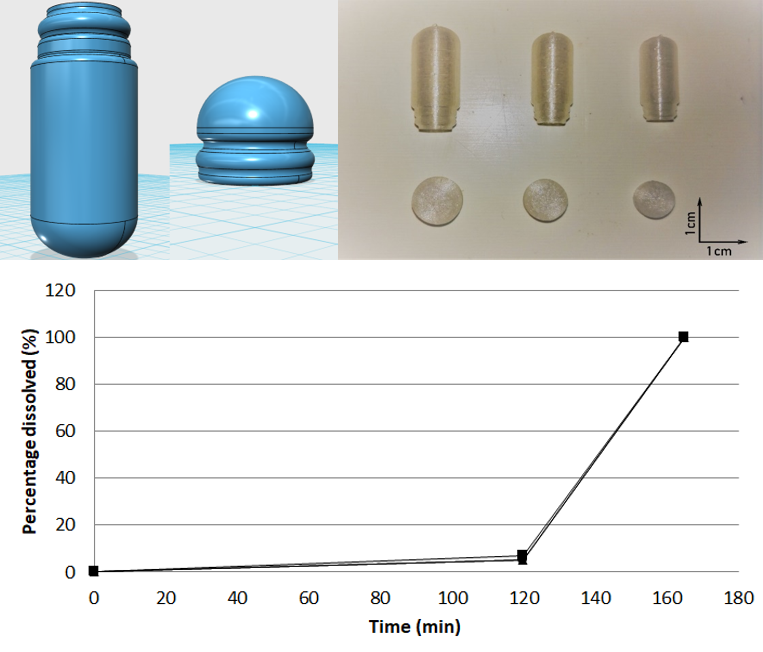
Investigation of the feasibility to manufacture enteric capsules, which could be used in compounding pharmacies, by fused-deposition modeling. It was demonstrated that our 3D printed enteric capsules did not release the drug for 2 h in acid medium (pH 1.2). However, they completely dissolved within 45 min at pH 6.8 which allowed the release of a minimal amount of 85% w/w of drug as it was recommended by the European Pharmacopoeia 9th Edition for enteric products.
Potential application
- Caps delivery
Key advantage of the technology
- More robust process – less erratic in term of efficacy
- One-step process
- Automatic process
- No use of organic solvents
- Ability to prepare magistral preparations as well as small batches for pre-clinical studies with sustained-release properties
Technology Readiness Level

The laboratory
The Unit of Galenic Pharmacy and Biopharmacy (UPGB) has about 18 people, including 3 professors and 2 research technicians. The UPGB laboratory specializes in the preformulation and development of innovative pharmaceutical dosage forms, as well as in the evaluation of the bioavailability of finished products that can be delivered by oral, parenteral, pulmonary or nasal route. In addition, it hosts the spin-off InhaTaget Therapeutics on its premises, the activities of which are based on the Unit's research in the field of inhaled oncology. The UPGB laboratory is very active in the formulation of therapeutic macromolecules (eg peptides, antibodies). He also focuses on the development of new drug production processes, including 3D printing. Another second major line of research of the UPGBu laboratory focuses on the administration of pulmonary drugs by seeing inhaled (pulmonary or nasal delivery), from the development of innovative formulations to their production. The different technologies used are based on particle engineering, evolving techniques (high pressure homogenization or spray drying) or conventional mixing methods.
Keywords
- 3D printing
- Caps
- Delivery systems
Collaboration type
- Licence agreement
- R&D collaboration
IP status
- Priority data: EP19166490.3 filed on April 1st, 2019
- Patent pending in Europe
Inventors
- Pr Jonathan Goole
Contact
ULB Research Department
Joachim Ruol
Business developer
+32 (0)2 650 30 67
joachim.ruol@ulb.be